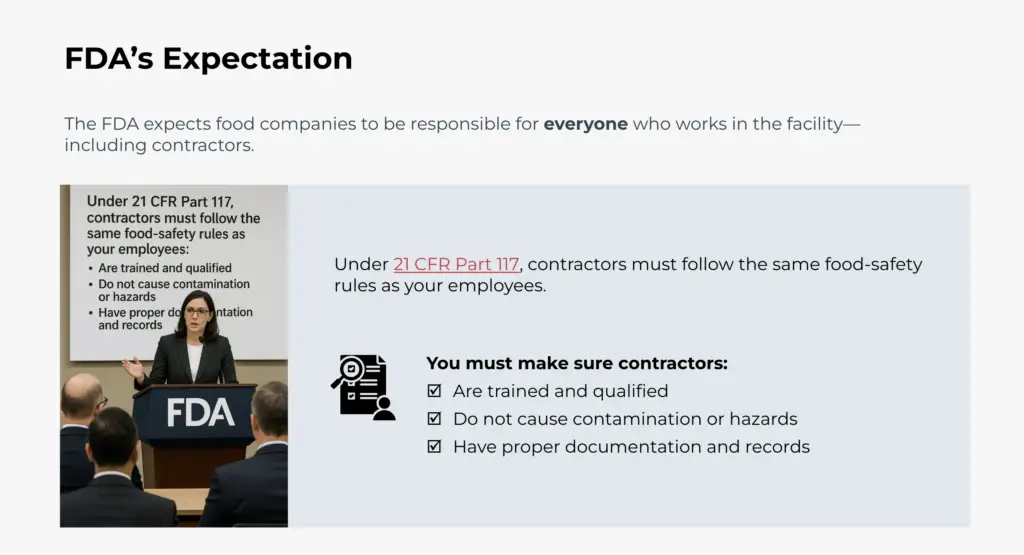
Contractor activities within food facilities require defined controls to ensure alignment with food safety programs and regulatory requirements.
This course focuses on facility-level contractor control programs, including contractor approval and pre-qualification, documentation requirements, GMP expectations, supervision, and verification of contractor performance. Emphasis is placed on program structure, oversight, and record-keeping to support consistent implementation and audit readiness.
The course is intended for professionals responsible for establishing, managing, and maintaining contractor control programs within food production environments.
After completing this course, participants will be able to:


To register for this course, please visit our training portal.

Dr. Suf Alkhaldi joined IEH as the Senior Vice President of Technical Services in 2023. He earned his M.S. and Ph.D. in Microbiology and Cell Molecular Biology from Oklahoma State University. He also conducted a postdoctoral study in Rumen Microbiology in Animal and Dairy Science at the University of Georgia and served as the manager of the DNA Microarray Lab at Yerkes National Primate Research Center at Emory University.
Before joining IEH, Dr. Alkhaldi spent over two decades at FDA which has been characterized by his exceptional contributions to innovation and regulatory excellence. He began as a researcher and food outbreak investigator at the Center for Food and Applied Nutrition (CFSAN), focusing on developing cutting-edge methods for identifying pathogenic bacteria in food using molecular techniques. With over 29 peer-reviewed manuscripts, 14 reviewed papers and book chapters, and co-editing “The Bad Bug Book,” he demonstrated a strong commitment to knowledge-sharing and food safety. Dr. Alkhaldi has played an instrumental role in resolving the FDA’s 2008 pepper outbreak investigation.
Throughout his extensive career at FDA, Dr. Alkhaldi held various roles, including Project Manager, Supervisor, and Senior Science Advisor in the Office of the Chief Scientist. He actively participated in influential working groups and the FDA Funded Centers of Excellence, reinforcing his dedication to advancing microbiology and FDA drug policies. Adding to his accomplishments, Dr. Alkhaldi received over 20 FDA awards between 2000 and 2023.
Dr. Alkhaldi was selected to lead the Office of Safety within the Office of Regulatory Affairs (ORA). His exceptional leadership during the COVID-19 pandemic was evident as he led a team of 24 Industrial Hygienists and safety professionals. Their dedication ensured the safety of 5,000 FDA employees and provided support for 16 FDA laboratories, demonstrating his significant impact in fostering a safe and innovative regulatory environment within the FDA and the broader field of food microbiology.
SELF-PACED ELEARNING
1 HOUR